Kidney+Neuro exam preparation
Previous exam 2023
Questions 1-9 Neuro
Questions 10-17 Kidney
1) Information or resources Define the concept of “window of susceptibility”. (2p)
1 | Windows of susceptibility refer to specific periods in an individual's life when they are more vulnerable to the effects of environmental health hazards. |
From https://library.fiveable.me/key-terms/environmental-occupational-health/windows-of-susceptibility
1 | Toxicants have different effects based on the timing of exposure during development and sensitivity changes with the development stage. this is called windows of susceptibility. |
From Rishi’s note, which is from tutor’s saying.
2) Neurotoxin Y blocks the Na/K ATP-ase. (2p)
3) Why is the nervous system more sensitive to neurotoxicants during development as compared to the adult state? Describe three specific features and motivate. (3p)
1 | The immature NS is especially vulnerable to certain chemicals, and there are several factors that make the developing NS uniquely susceptible. |
From text book page 844. Summarized by ChatGPT.
4) Describe three neurotoxic mechanisms which can account for the developmental neurotoxicity of methylmercury (MeHg). (3p)
1 | 1. Oxidative Stress: Methylmercury (MeHg) increases reactive oxygen species, damaging neural cells during critical developmental stages. |
Answered by ChatGPT based on points from slides.
5) One of the following is NOT relevant for methyl mercury (MeHg) neurotoxicity. (1p) Select one alternative:
- increase in intracellular Calcium
- interference with glucocorticoid hormone signalling
- interference with thyroid hormone signalling
- oxidative stress
6a) Define excitotoxicity. (3p) 6b) Describe two circumstances which promote excitotoxicity.
1 | Excitotoxicity is a condition of central neuronal death due to an excess of glutamate or related excitatory molecules binding to their respective receptors. |
From https://www.sciencedirect.com/science/article/pii/S1054358916300096
7) Tetrodotoxin (TTx) is a neurotoxin which blocks voltage-gated Na channels (prevents them from opening). (2p)
7a) What effect does TTx have on resting membrane potential?
7b) What effect does TTx have on action potential?
1 | 7a) Effect on Resting Membrane Potential: Tetrodotoxin (TTx) does not affect the resting membrane potential because it only blocks voltage-gated sodium channels, which are not active at rest. |
8) Which symptoms are associated with sarin gas intoxication? Select one or more alternatives:
- paralysis of skeletal muscles
- euphoria
- excessive salivation
- excessive sweating
9) Define the concept of “silent toxicity”. (2p)
1 | “Silent neurotoxicity”, also called silent toxicity or silent damage to the nervous system, represents a persistent biochemical change or morphological injury to the nervous system that does not induce overt evidence of toxicity (i.e. remains clinically unapparent) unless unmasked by experimental or natural processes. |
From https://www.sciencedirect.com/science/article/pii/S0892036216300162
10) What are the three main functions of the kidney and give one concrete example of each function. (3p)
1 | 1. Regulation of Blood: The kidney regulates blood pressure and electrolyte balance (e.g., adjusts sodium and water levels to control blood pressure). |
Points from slides and concreted by ChatGPT.
11) The renal tubule has three different sections, which are these and in what order do they appear? (2p)
1 | The three sections of the renal tubule, in order, are: |
From slides
12) The kidney secretes several hormones, name two of these hormones and explain one main function for each respective hormone. (2p)
1 | Renin activates the renin-angiotensin-aldosterone system, thus |
From slides
13) Mention two types of clinical tests that can be performed to detect glomerular and tubular damage, respectively. (2p)
1 | Glomerular damage: |
From slides
14) Give examples of two diuretic substances (natural or drug), and explain how they affect diuresis? (2p)
1 | 1. Caffeine. Caffeine can inhibits Na+ reabsorption |
From slides
15) Development of chronic renal failure occurs progressively. (4p) a. What defines the last stage, referred to as end-stage renal disease? b. Give one example of a substance that has been associated with chronic kidney disease (CKD). c. Briefly describe one underlying mechanism by which your selected substance can cause kidney damage.
1 | a. |
From slides
16) Give two examples of occupations where individuals may be exposed to nephrotoxic substances, and state one substance for each occupational exposure and the main site of action for each respective substance in the kidney. (3p)
1 | 1.Electronic waste recycler; Cadmium; proximal tubular |
From PBL and slides.
17) What does the half-life of a drug mean and what are the two factors that the half-life is dependent on? Also, give one example of how the half-life of a drug can be affected by the presence of kidney failure? (2p)
1 | Half-life (t1/2): The time it takes for the concentration of a chemical in the body to decrease by half. |
Definition and factors from slides. Example from slides and refined by ChatGPT.
Points from slides(Kidney)
Kidney structure
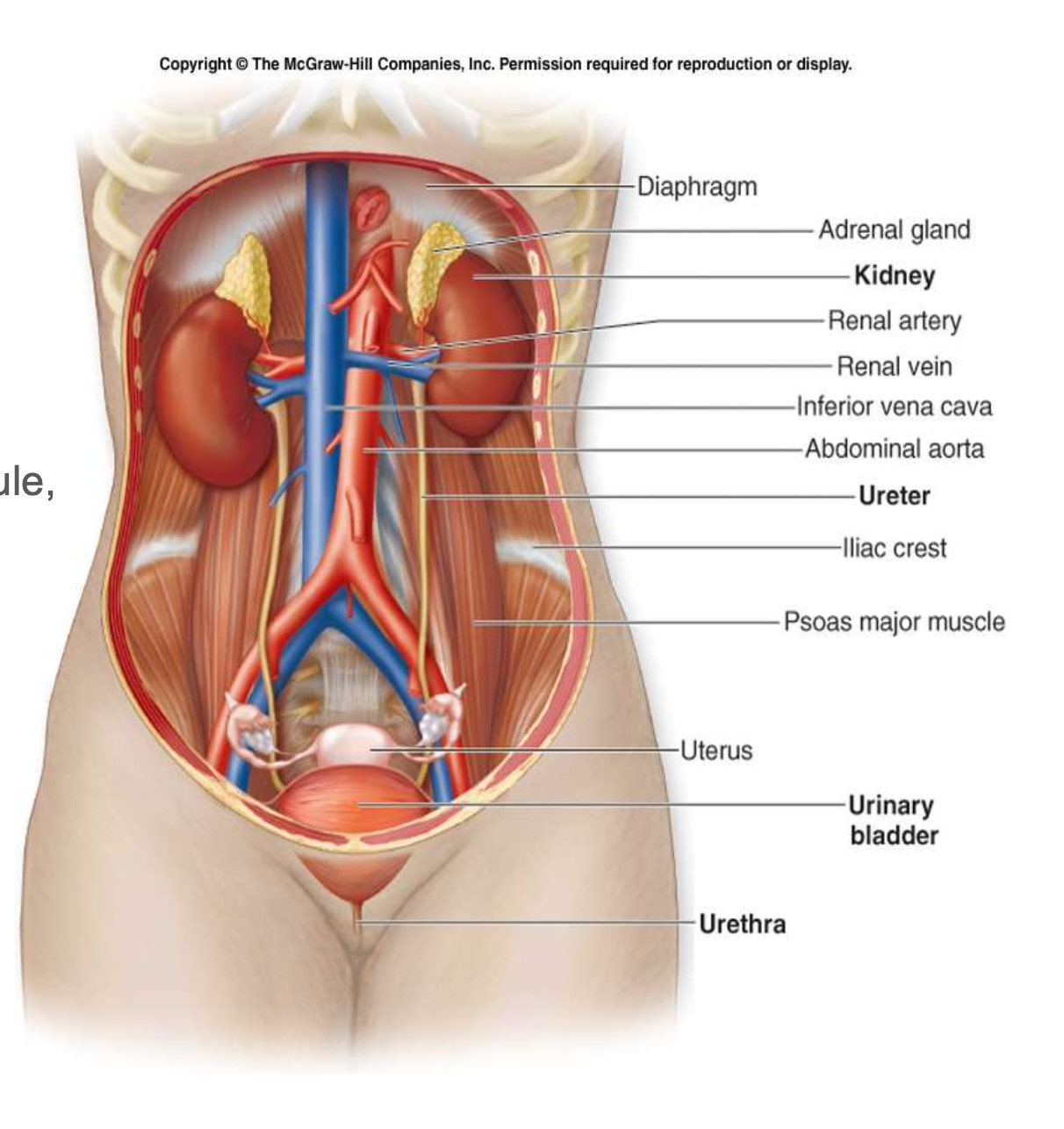
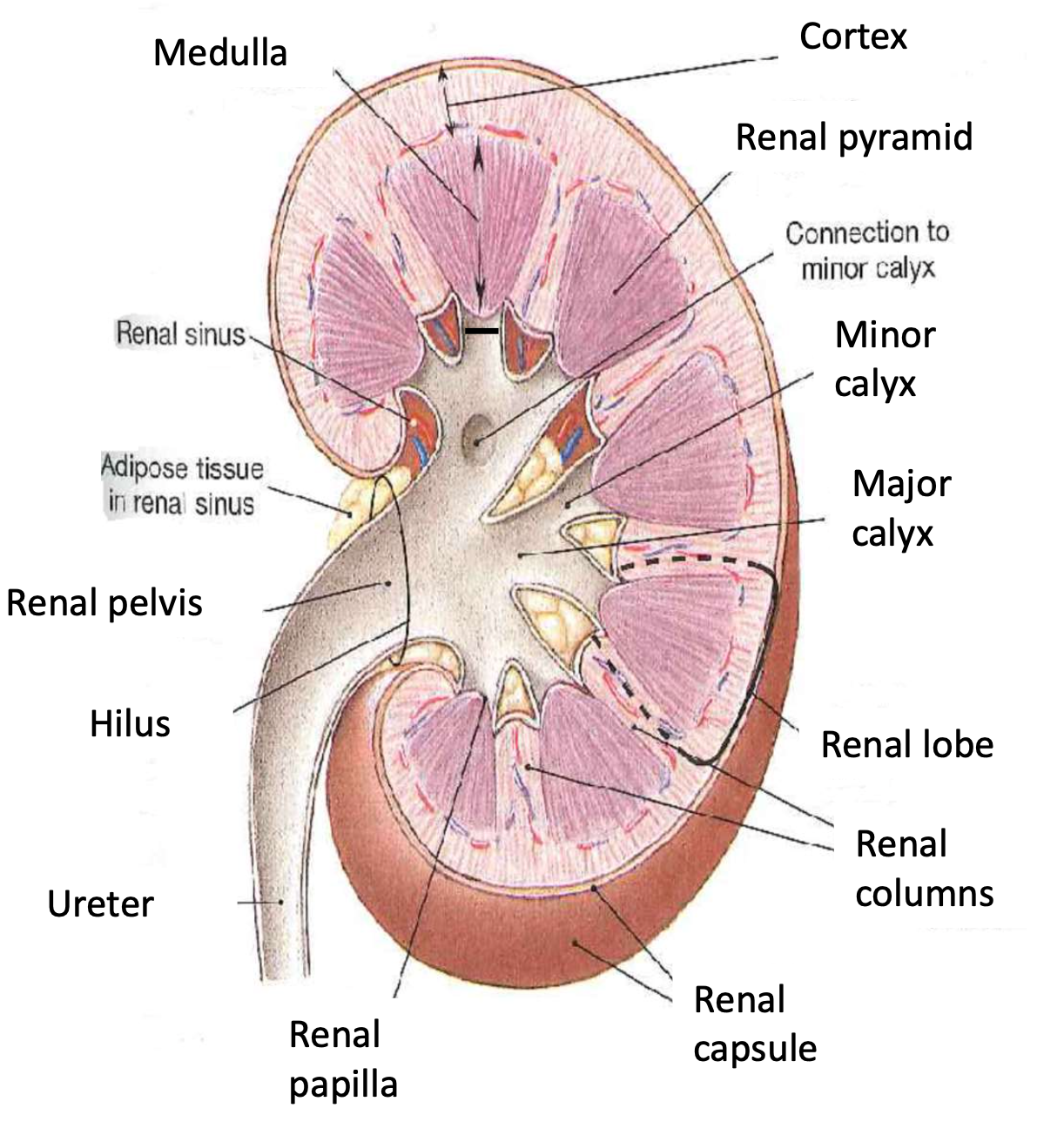
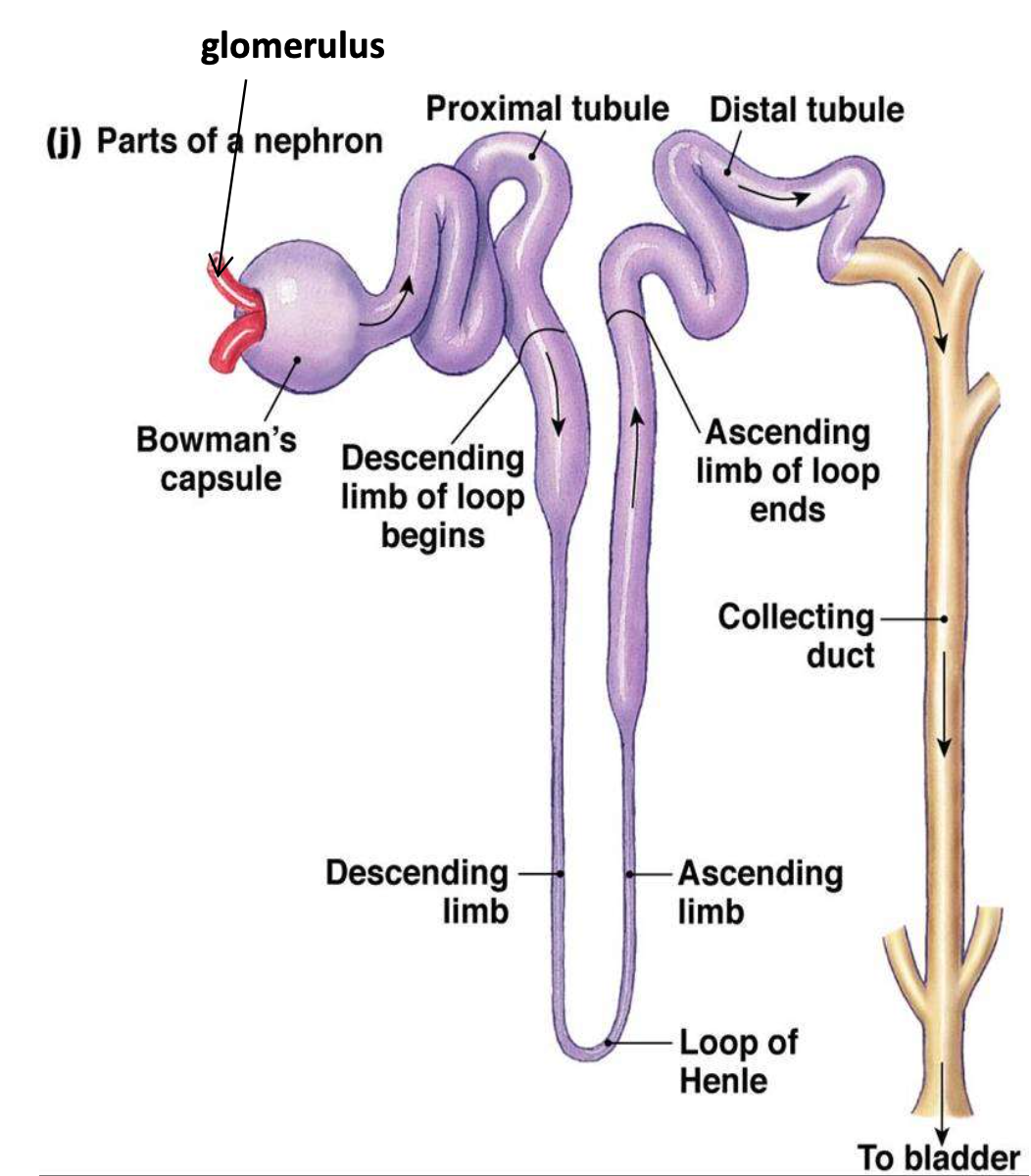
Order of urine production
Nephron => papillary ducts=> minor calyces => major calyces => renal pelvis => ureter => urine bladder
nephron
TWO parts:
- Renal corpuscle
- Glomerulus - capillary network
- Bowman’s capsule – double-walled cup surrounding the glomerular capillaries
- Renal tubule
- Proximal convoluted tubule
- Loop of Henle
- Distal convoluted tubule
Urine production
- GOAL: to maintain homeostasis by regulating the volume and composition of blood.
- Involves excretion of solutes, especially waste products:
- Ammonia and urea: breakdown of amino acids (most abundant).
- Creatinine: breakdown of creatinine phosphate, important role in muscle contraction.
- Uric acid: recycling of nitrogenous bases from RNA molecules.
Accomplished by filtration, reabsorption, secretion and excretion.
Rate of urinary excretion: glomerular filtration + secretion - reabsorption
Tubular reabsorption and secretion
- most reabsorption occur in proximal convoluted tubule
Glomerular filtration rate (GFR)
Is the amount of filtrate formed in all renal corpuscles of both kidneys each minute
Controlling GFR
Auto-regulation
- Myogenic mechanism (fast): ↑ blood pressure => stretching of smooth muscle in afferent arteriole wall => contraction => narrows lumen => ↓GFR
- Tubuloglomerular feedback (slower): ↑ blood pressure => less reabsorption of Na+, Cl- and water => detected by macula densa cells => ↓ release of NO (vasodilation) by juxtaglomerular apparatus => contraction of afferent arteriole = ↓GFR
Neural regulation
- Sympathetic stimulation (exercise or hemorrhage) => release of norepinephrine => causes powerful vasoconstriction of afferent arterioles = ↓GFR.
Hormonal regulation
- Angiotensin II: potent vasoconstrictor => narrows both afferent and efferent arterioles => ↓ renal blood flow => ↓GFR.
- Atrial natriuretic peptide (ANP): secreted by the heart at ↑ blood volume => ↑ capillary surface => ↑GFR
Renin – Angiotensin – Aldosterone System
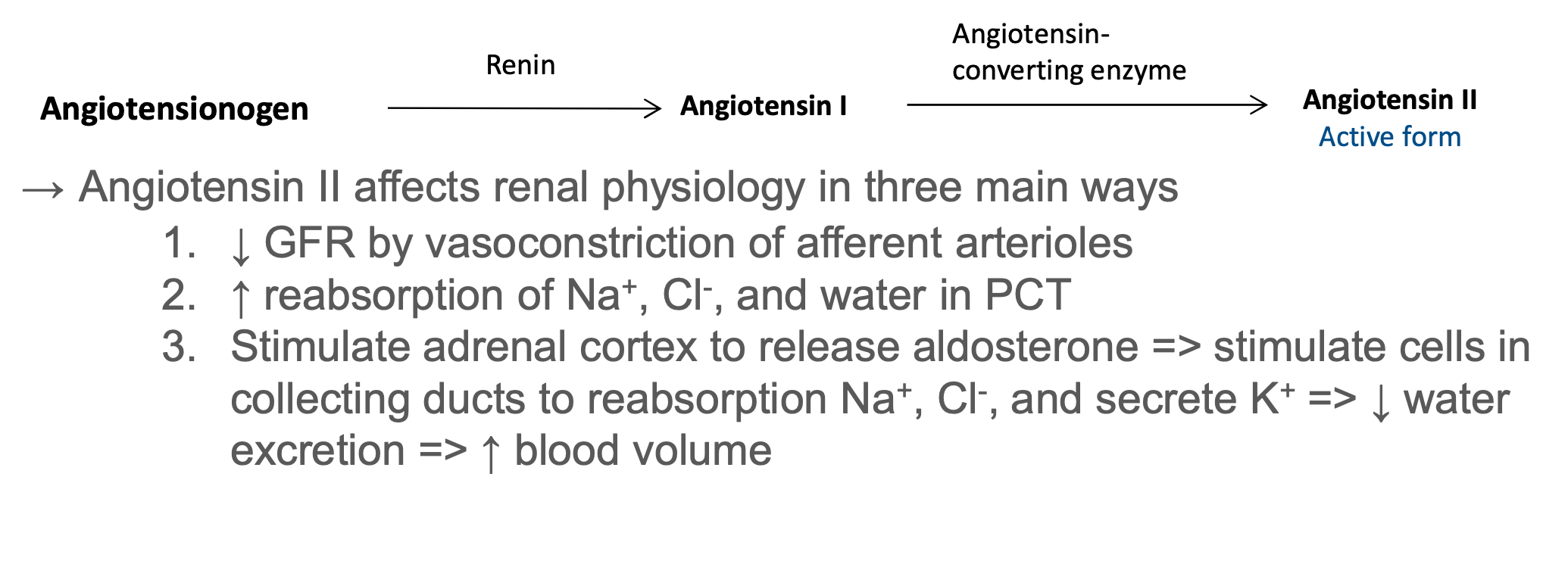
Hormone production
- Renin activates the renin-angiotensin-aldosterone system, thus regulating blood pressure and Na+,K+ balance
- Prostaglandins/kinins bradykinin = vasoactive, leading to modulation of renal blood flow & along with angiotensin II affect the systemic blood flow
- Erythropoietin stimulates red blood cell formation by bone marrow
Effects to ADME when kidney failure occurs
- Absorption and bioavailability
In case of kidney failure, the motility of the stomach is decreased and intestinal edema may occur, decreasing the absorption of drugs such as the diuretic furosemide.
- Distribution and protein binding
In case of kidney failure, the protein binding of drugs is decreased mainly due to lower albumin concentrations in plasma moving the drug from the blood to organs, increasing the distribution of the drug. This leads to a decreased plasma concentration of such drugs but increased concentrations of the active form in organs. This in turn increases the half-life of the drug. Examples of such drugs are warfarin, phenytoin and morphine.
- Elimination
Elimination through the kidneys is mostly dependent on the eGFR. In case of decreased eGFR, the half-life of a drug increases
Toxicants lead to kidney failure
NSAIDs
NSAID can cause a decreased perfusion to the kidneys and decrease the eGFR in case of decreased blood pressure/hypovolemia, dehydration, heart failure.
ACE-inhibitors(Angiotensin converting enzyme inhibitors)
ACE-inhibitors can decrease the eGFR through a decrease of the glomerular capilar pressure.
Metformin
Points from slides(Neuro)
Nervous system anatomy
- CNS (central nervous system)
- PNS (peripheral nervous system)
- Somatic nervous system
- Autonomous nervous system
- parasympathetic nerves
- sympathetic nerves
Action potential
- Resting membrane potential
- Depolarization (Na channels open)
- Repolarization (K channels open)
- Refractory period (Na/K pump re-establish membrane potential
Neurotoxicity Definition
Adverse change in the structure or function of the central or peripheral nervous system following exposure to a chemical or physical agent.
Neurological deficit Definition
Functional abnormality of a body area due to decrease in the function of the brain, spinal cord, nerves, or muscles.
How signaling across the synapse
Synaptic signaling between neurons involves diffusible chemical messengers (neurotransmitters) released by the presynaptic neuron into the synaptic cleft Neurotransmitters bind to receptors on the postsynaptic neuron and activate ion channels (ionotropic receptors) → local changes in membrane potential → action potential → propagation along the axon
Indirect neurotoxicity definition
neurologic alterations due to agents acting primarily on sites outside the nervous system(eg. CO; EDCs)
adult vs. developing nervous system
| feature | adult CNS | developing CNS |
|---|---|---|
| state | static (?) | dynamic |
| main processes | electric signals; plasticity | proliferation and differentiation (plasticity) |
| functional reserve | large | small |
| structural reserve | small | large |
| damage | cell death; | altered progenitor proliferation and differentiation; reprogramming |